By Krishna N. Das, Praveen Paramasivam and Jennifer Rigby
NEW DELHI/CHENNAI (Reuters) -Indian authorities advised the public to avoid two more brands of cough syrup on Wednesday following the deaths of 17 children aged under five linked to a toxic ingredient, as the World Health Organization said the country had a “regulatory gap” in screening locally-sold syrup medicines.
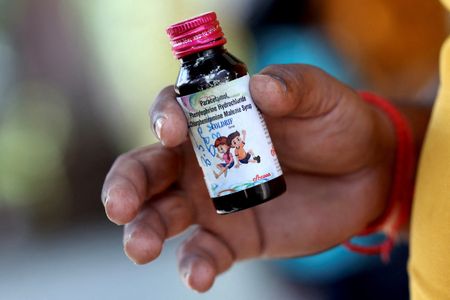
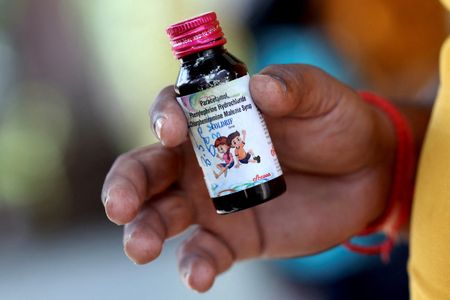
The children died in India over the past month after consuming cough medicine containing toxic diethylene glycol in quantities nearly 500 times the permissible limit, officials say. The deaths were all linked to the Coldrif medicine, banned after a test confirmed the presence of the chemical on October 2.
The Respifresh and RELIFE syrups also contain diethylene glycol, according to a public alert by Gujarat and other states on Wednesday that described it as “a toxic chemical that can cause serious poisoning, including kidney failure, neurological complications and even death, especially among children”.
The World Health Organization told Reuters it had received confirmation from India on Wednesday that three contaminated syrups had been identified, and none had been exported.
WHO ADVISES AGAINST COUGH AND COLD MEDICINES FOR CHILDREN
However, the U.N. health agency urged caution as it was possible some exports had taken place unofficially, and the source of the contamination had not yet been found.
“WHO expresses deep concern over these developments and emphasizes… the regulatory gap in DEG/EG screening for domestically marketed medicines in India,” a spokesperson added by email, referring to diethylene glycol and ethylene glycol.
By law, Indian drugmakers must test each batch of raw materials and the final product. Exports of cough syrups have required another layer of tests at government-mandated laboratories since 2023 following the deaths of over 140 children in Gambia, Uzbekistan and Cameroon linked to Indian syrups.
Coldrif, made by Sresan Pharmaceutical Manufacturer, was only sold locally, according to a government document seen by Reuters. Gujarat officials said the other two syrups were sold in other Indian states but did not refer to exports.
The companies and drug officials did not respond to questions over whether the other two syrups, RELIFE made by Shape Pharma and Respifresh made by Rednex Pharmaceuticals, were exported as well.
The WHO said earlier it would assess the need for a Global Medical Products Alert on Coldrif syrup once it received official confirmation from Indian authorities.
The U.N. health agency is continuing to advise against the use of cough and cold medicines for children.
Earlier the country’s drug controller general, Rajeev Raghuvanshi, said the regulator had found serious lapses at factories making the drugs in checks that showed they failed to test every batch of medicinal ingredients as required.
In the advisory dated October 7 and posted on a government website, Raghuvanshi did not name any companies or the number of them that were found to have flouted rules, but said the inspections had been carried out at firms whose drugs had earlier been found to be below standard quality.
ABANDONED FACTORY
Reuters could not contact Sresan chief G. Ranganathan, whose office and factory in the southern state of Tamil Nadu were shut. Police are investigating the company for manslaughter, sales of the syrup have been banned, and central authorities have recommended cancelling Sresan’s manufacturing licence.
Reuters saw drug inspectors pasting a notice on Tuesday on the peeling walls of the Sresan facility, in the industrial district of Kancheepuram, Tamil Nadu, that asked for details including how the medicine was produced and where its ingredients were sourced.
There was no one present to answer the officials. Behind the facility, syrup bottles and burnt medicines lay strewn across the ground, with a sharp chemical odor lingering in the air.
The health ministry said on Sunday authorities were carrying out inspections across 19 other manufacturing units in six states.
Two of the inspected companies were Shape Pharma and Rednex Pharmaceuticals, based in Gujarat, a key pharmaceutical manufacturing hub. State authorities said on Tuesday that samples of cough syrups produced by the companies had been found to be “not of standard quality”.
State and federal inspectors identified unspecified deficiencies and ordered an immediate halt to all production and distribution. Shape and Rednex did not respond to requests for comment.
Ethylene or diethylene glycol toxins were found in Indian-made cough syrups that killed children in Gambia, Uzbekistan and Cameroon since 2022, and 12 children in India in 2019, damaging the image of the world’s third-biggest drug-manufacturing country by volume.
India’s pharmaceutical industry, exceeded in size only by the U.S. and China, is valued at $50 billion. More than half of its value comes from exports.
India supplies 40% of generic medicines used in the U.S., and more than 90% of all medicines in many African nations.
(Additional reporting by Sumit Khanna in Ahmedabad, Praveen Paramasivam in Chennai, Jennifer Rigby in London and Sudipto Ganguly in Mumbai; Writing by Krishna N. Das and Philippa Fletcher; Editing by Raju Gopalakrishnan, Ed Osmond, Alexandra Hudson and Nia Williams)